Example of AFFF residue inside an AFFF foam tank after initial AFFF draining. The residue is difficult to remove using water alone but dissolves readily in heated PerfluorAdTM solutions.
Aqueous film forming foam (AFFF) formulations, used for rapidly extinguishing fuel and oil fires, contain up to 6% per- and polyfluoroalkyl substances (PFAS). As state and federal agencies are limiting the allowable PFAS concentrations in the environment, the firefighting community is about to undergo the massive process of removing AFFF from firefighting systems (mobile and fixed) and replacing them with fluorine free foams (FFF). This effort will not only include draining AFFF from these systems but will also involve cleaning their wetted surfaces.
Unfortunately, PFAS form coatings that are resistant to removal and do not dissolve readily in water. Multiple studies have shown that simple rinsing with water leaves large quantities of PFAS behind (Lang et al. 2022).
Fortunately, PerfluorAd, a plant-based, weak acid, can remove PFAS far more efficiently than water.
Triple Rinse v. PerfluorAd
The steps of AFFF removal and cleanout follow:
- Removal of AFFF concentrate
- Physical rinsing of foam tanks, pipes, and venturies
- Rinsing of the wetted surfaces using a suitable liquid
- Treatment or disposal of the rinsate and other generated waste
TRS Group (TRS) recently compared the effectiveness of triple-rinse (three rinses of the AFFF tank using potable water only) to rinsing with a PerfluorAdTM solution. We cleaned a firefighting vehicle at a major international airport using established triple-rinse methodology. Then, we treated the same vehicle using a heated PerfluorAdTM solution with flushing and agitation, rinsing the vehicle’s internal surfaces, much like the action inside a dishwasher.
After each cleaning, we filled the foam tank with water, circulated it throughout the system, let it equilibrate, and then took samples, analyzing for specific PFAS compounds.
Figure 1 below shows PFAS concentrations found after rinsing with water and PerfluorAd.
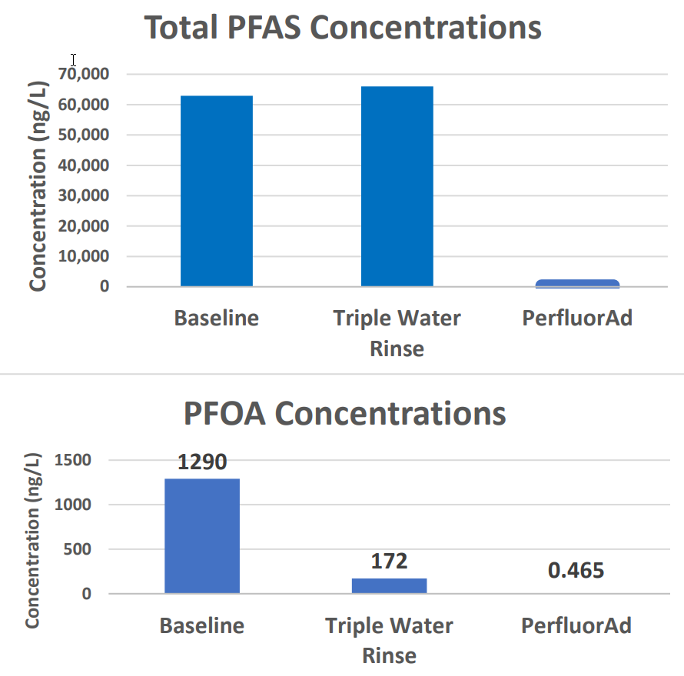
Figure 1. PFAS concentrations before and after triple rinse and PerfluorAdTM rinsing.
Note that the triple-rinse reduced the concentration of several PFAS compounds, such as PFOA, in the vehicle’s AFFF storage tank; however, there was no reduction in total PFAS concentrations. The dominant compound in the triple-rinsed water was 6:2 fluorotelomer sulfonic acid (6:2 FTS), which actually increased after rinsing with water. In contrast, PerfluorAdTM flushing resulted in a reduction of greater than 99 percent for all PFAS compounds. Furthermore, we saw 99.3% removal for 6:2 FTS and non-detect levels for PFOA and PFOS.
A significant advantage of PerfluorAd is it dramatically reduces the volume of effluent wastewater. PerfluorAd concentrates the PFAS by flocculation, reducing disposal costs significantly.
Applying the PerfluorAdTM process to fire suppression systems can lead to PFAS reductions of more than 99 percent. Triple-rinsing with water resulted in some PFAS reductions, but with an insignificant change in total PFAS concentrations. PFAS-containing residues inside the vehicles cannot be removed effectively using water alone.
Reference:
Johnsie R. Lang, Jeffery McDonough, T.C. Guillette, Peter Storch, John Anderson, David Liles, Robert Prigge, Jonathan A.L. Miles, Craig Divine (2022): Characterization of per- and polyfluoroalkyl substances on fire suppression system piping and optimization of removal methods. Chemosphere 308, 136254.





